Oncovita is the owner of a disruptive virus vector technology to produce new immunotherapy treatment against cancer. Derived from the safe and highly immunogenic measles vaccine virus with clinically proven oncolytic potential, the MeasovirTM platform has a great number of advantages: not only does the virus specifically target and destroy cancer cells, but it also has the potential to elicit an effective and long-lasting anti-tumor immunity. The measles vaccine virus is naturally oncolytic with a high degree of specificity for cancer cells due to the overexpression of its entry receptor CD46 on the surface of cancer cells. CD46 is an inhibitory receptor of the complement used by cancer cells as a defense against attacks from the host immune system. The MVdeltaC virus developed by Oncovita has an increased power to induce immunogenic cancer cell death through its strong capacity to activate RIG-I, a cytosolic RNA receptor recently demonstrated as critical for responsiveness to checkpoint blockade in anticancer therapies.
Immuno-oncolytic viruses
Cancer cell and T cells
Engineered viruses have proven to be powerful “bullets” to target cancer cells and to promote their immunogenic death. Oncolytic viruses are replicative viruses that preferentially infect tumor cells while preserving healthy cells. Some of them, in particular the measles virus, exhibit immuno-adjuvant properties that reinforce the antitumor immune response by breaking immune tolerance to the cancer. The potential advantages of oncovirotherapy over conventional cancer treatments include:
- higher on-target specificity against cancer allowing a better safety margin
- bypassing resistance to cell death induction in aggressive cancers
- long-lasting effects due to immune memory activation to prevent relapse and metastasis
Measles vaccine
Measles live attenuated virus is one of the safest and most efficacious human vaccines that saves 2-3 million babies’ lives each year. The virus was attenuated in the 60s by serial passages on chicken cells giving rise to several attenuated strains among which the Schwarz strain is the most attenuated and widely used in human preventive medicine
- One low-dose injection
- Life-long protective immunity
- Safety / efficacy track record over 2 billion children
- High genetic stability
- Established manufacturing
This vaccine is given to 80 – 95% of newborns worldwide (100 million babies per year). It induces life-long protective immunity after a single dose and a booster dose is recommended. Its safety and efficacy track record has been recorded in more than 2 billion children over the last 40 years. It is manufactured with well-established processes and does not contain adjuvant. As a paramyxovirus with non-segmented negative stranded RNA genome, it benefits of a high genetic stability.
MeasovirTM a unique platform to kill cancer cells and activate immune system
Oncovita’s MeasovirTM proprietary platform relies on a DNA plasmid vector that expresses a modified measles Schwarz virus antigenome able to generate recombinant viruses by using a clonal reverse genetics system. This versatile plug-and-play platform allows producing new viruses expressing very large amounts of additional genetic material.
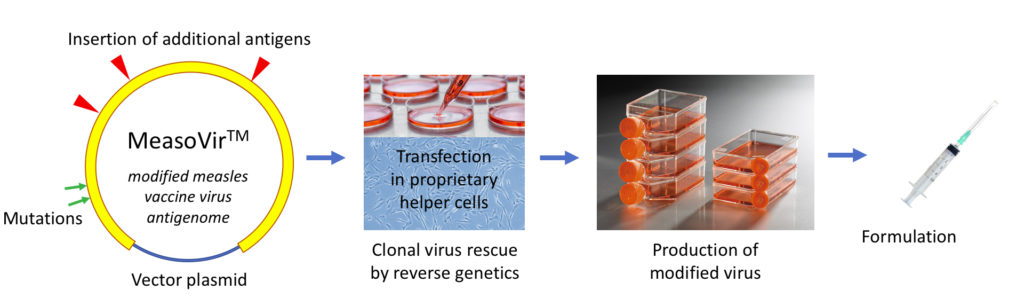
MeasovirTM has been constructed by deletion of a viral factor which normally controls apoptosis of infected cells. Its increased capacity to induce immunogenic cancer cell death is linked to its strong RIG-I agonist activity (RIG-I is a cytoplasmic RNA receptor whose activation is critical for responsiveness to checkpoint blockade in cancer). MeasovirTM platform has been patented (granted in US and Europe for treatment of all types of cancers).
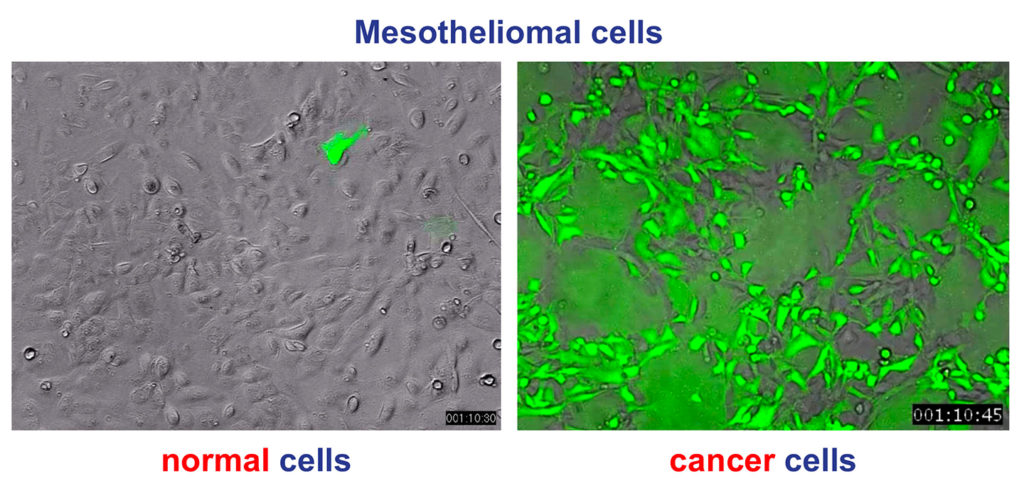
Unique specificity for cancer cells: MeasovirTM efficiently targets and destroys cancer cells, while leaving healthy cells unaffected (the image shows the specificity of infection of cancer cells with MeasovirTM expressing the green fluorescent protein). MeasovirTM takes advantage of the natural property of measles vaccine to specifically infect cancer cells and arrest their immune-blocking activity. This property is related to overexpression of CD46 at the surface of cancer cells, a complement inhibitor that helps tumor cell survival by protecting them from attacks by the complement system and NK cells. CD46 being a specific entry receptor of measles vaccine virus, its overexpression is an Achilles heel of cancer cells. This makes measles vaccine virus naturally oncolytic.
The following video illustrates the cancer cells death mediated by MeasovirTM.
Cancer mesothelial cells infected by MeasovirTM (green)
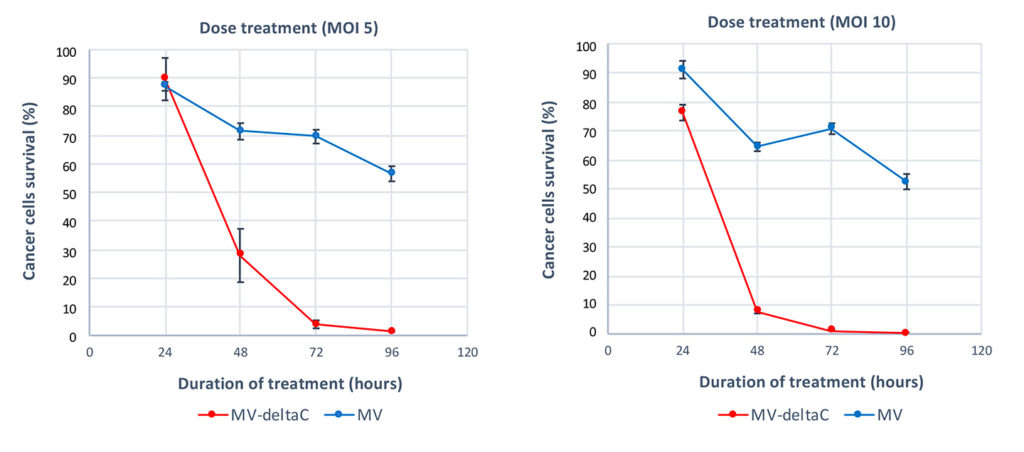
In vitro activity: In vitro preclinical studies demonstrated the strong specificity and high efficiency of MeasovirTM treatment in multiple cancer cell types including mesothelioma, bladder cancer, ovarian cancer, hepatocellular carcinoma, colon cancer, melanoma, and multiple myeloma. More than 100 cancer cell lines and primary cancer cells have been tested and more than 70% of them responded to treatment. This figure shows the strong advantage of modified MV-deltaC virus as compared to standard MV vaccine in killing cancer mesothelioma cells : MV-deltaC kills cancer cells more rapidly and more efficiently.
In vivo activity: In vivo tumor regression by MeasovirTM treatment has been demonstrated in nude and NOD-SCID mice engrafted with several human cancer cells.
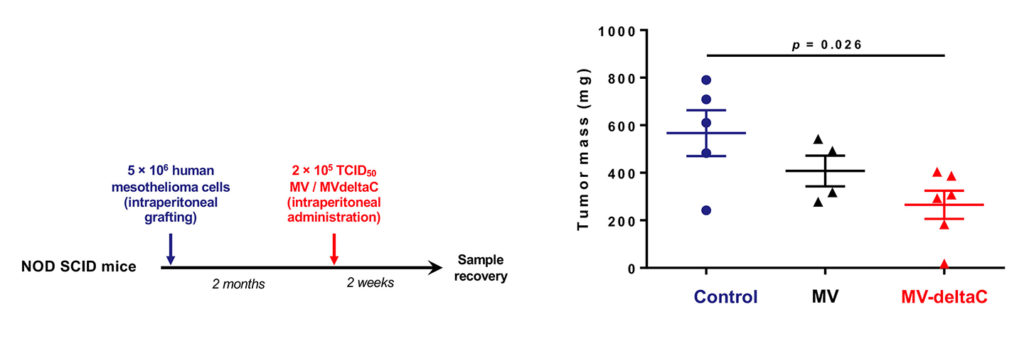
Here is shown the higher efficiency of MV-deltaC virus as compared to standard MV vaccine to eliminate tumors in an orthoptic xenograft model after a single dose treatment. NOD-SCID mice were grafted with human mesothelioma cells then treated 2 months after with a very low dose treatment administered intraperitoneally.
MVdeltaC antitumor activity was also evaluated in vivo on mesothelioma PDX model in nude mice (EPO, Berlin). Results are very positive: the treatment is active to stop tumor growth and also to reduce its size:
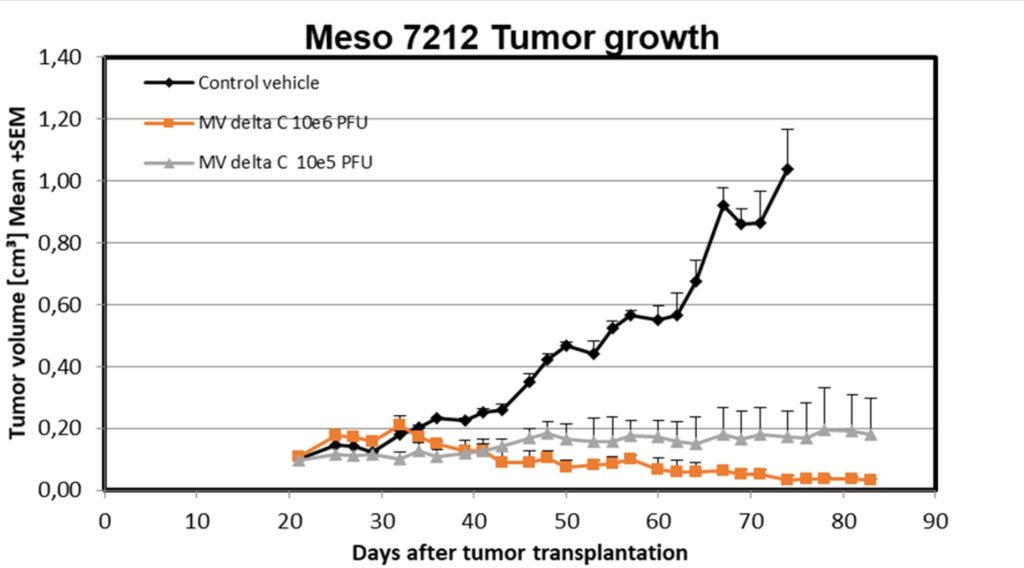
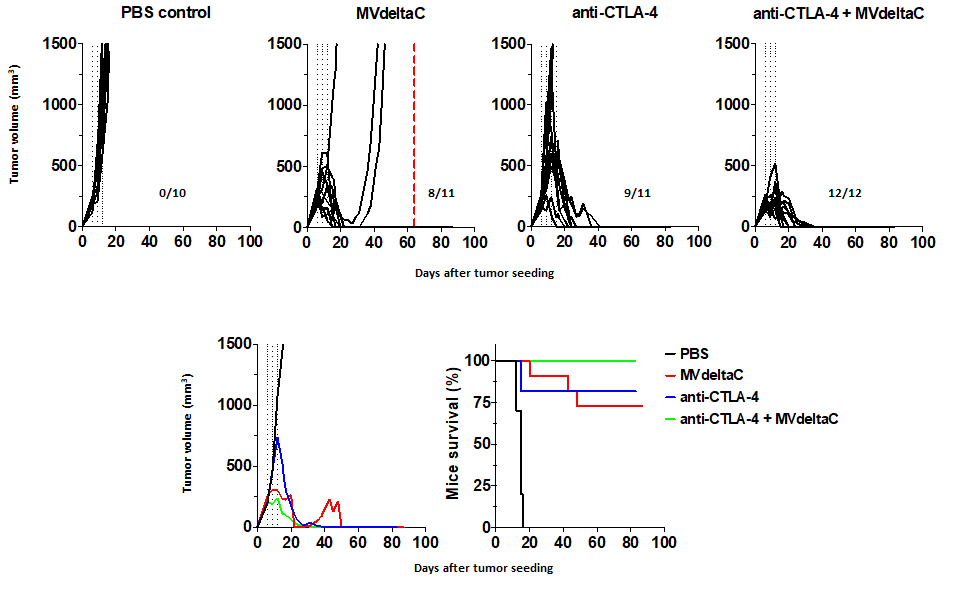
In-vivo proof of activity in immuno-competent mice
An even stronger anti-tumor activity was observed in a syngeneic model with immunocompetent mice (NS20Y tumors in A/J mice). A strong inhibition of tumor growth was observed after only 3 i.t. treatments, resulting in complete disappearance of tumors corresponding to a complete response according to RECIST criteria, associated with a high survival rate (75%).
Synergy was observed in the presence of anti-CTL-A4 antibodies resulting in a 100% survival rate.
After 3 months, the mice having eliminated the tumors were re-grafted with the same tumor cells: no tumor growth was observed, demonstrating an anti-tumor immune memory
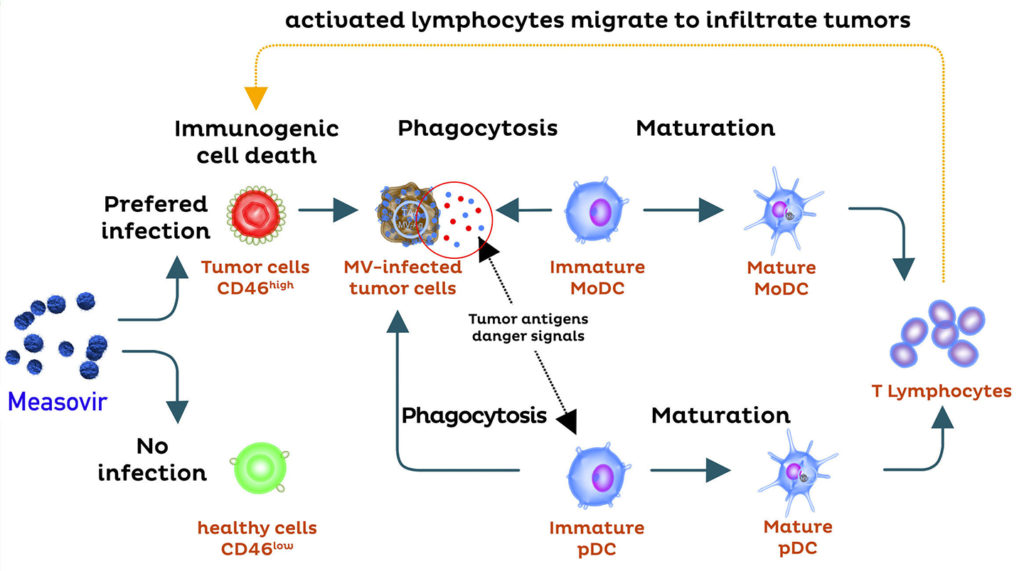
Immuno-oncolytic mode of action: The immuno-oncolytic mode of action of MeasovirTM treatment has been demonstrated by ex vivo experiments, in which human cancer cells treated with MeasovirTM were cocultured with human dendritic cells and T cells (see figure).
In this system, we demonstrated (i) the strong activation of both myeloid-derived and plasmacytoid-derived dendritic cells, (ii) the phagocytosis of dying cancer cells by dendritic cells, and (iii) the efficient cross presentation of specific tumor antigens to autologous T-cells. We also demonstrated the strong activation of RIG-I and cell death pathways and the production of large amounts of interferon α∫β.
MeasovirTM activates antitumor immune responses by inducing immunogenic death and breaking tumor tolerance by:
- rapid apoptosis of infected tumor cells
- expression of danger signals (hsP70, gp96, IFNα, IFNβ, hMGB1, IL-6, IL-8)
- activation and maturation of myeloid and plasmacytoid dendritic cells
- cross-presentation of tumor-associated antigens (TAA) to T cells
- killing of human tumors engrafted in mice
Mechanism of action in human cancer cells
All our in vitro and in vivo studies support the initiation of clinical trials to test the efficacy of MeasoVirTM against several types of tumors.
The core component of MeasovirTM – the measles vaccine virus – displays remarkably high safety records. Its industrial production is well established.
Therapy with Measovir
The core component of MeasovirTM has already demonstrated remarkable safety records. Its industrial production is well established and our studies support the initiation of clinical trials to test its efficacy against several types of tumors. Patients with solid or liquid tumors and with measles immune memory (90% of individuals have been vaccinated or infected) will be treated with repeated MeasovirTM administrations by intratumoral, peritumoral or systemic route. Infection kills cancer cells and activates measles immune memory in lymphoid organs. Activated immune cells (NK, CD4, CD8, B) migrate to infected cancer cells and help tumor clearance and memory establishment.
The following film illustrates the principle of this treatment.
Therapy with MeasovirTM